The Making of an Aurora
The Aurora Borealis (Northern Lights) and Aurora Australis (Southern Lights) are the result of electrons colliding with the upper reaches of Earth’s atmosphere. (Protons cause faint and diffuse aurora, usually not easily visible to the human eye.) The electrons are energized through acceleration processes in the downwind tail (night side) of the magnetosphere and at lower altitudes along auroral field lines. The accelerated electrons follow the magnetic field of Earth down to the Polar Regions where they collide with oxygen and nitrogen atoms and molecules in Earth’s upper atmosphere. In these collisions, the electrons transfer their energy to the atmosphere thus exciting the atoms and molecules to higher energy states. When they relax back down to lower energy states, they release their energy in the form of light. This is similar to how a neon light works. The aurora typically forms 80 to 500 km above Earth’s surface.
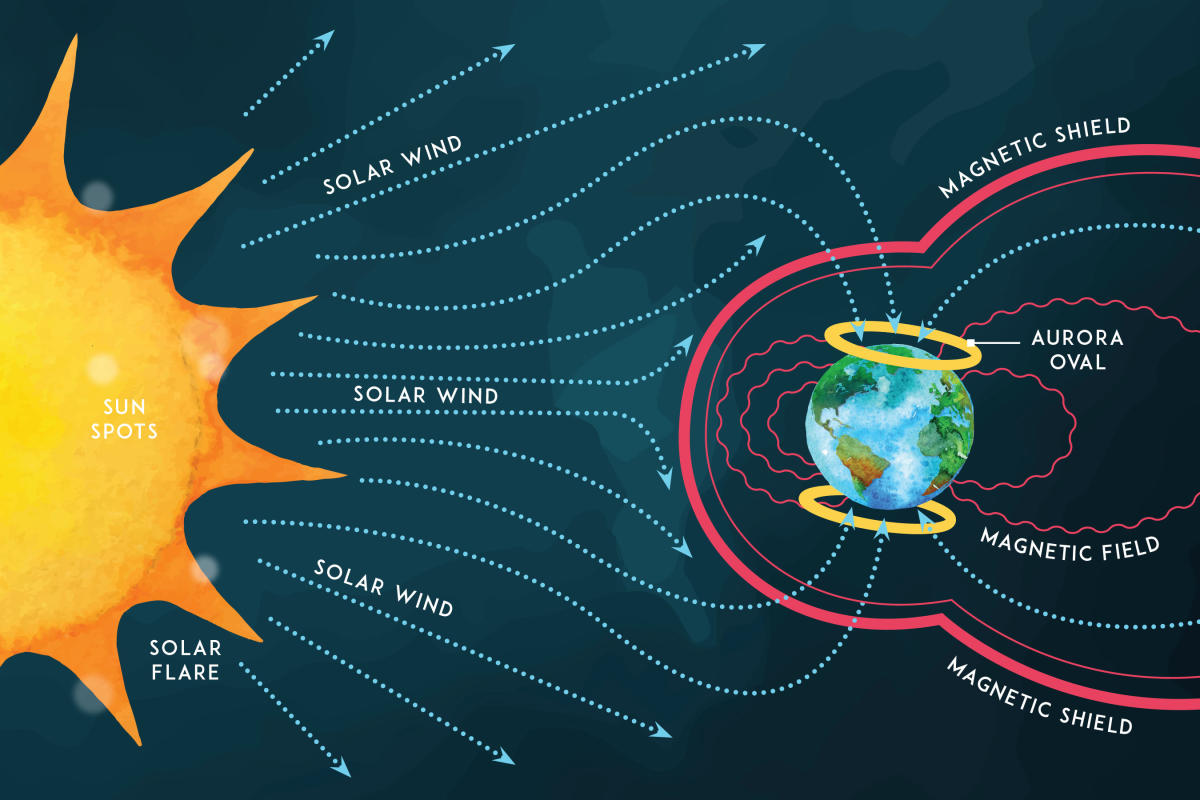
The Magnetosphere
Earth's magnetosphere is shaped by the impact of the solar wind on the Earth's magnetic field. This forms an obstacle to the flow, diverting it, at an average distance of about 70,000 km (11 Earth radii or Re), producing a bow shock 12,000 km to 15,000 km (1.9 to 2.4 Re) further upstream. The width of the magnetosphere abreast of Earth, is typically 190,000 km (30 Re), and on the night side a long "magnetotail" of stretched field lines extends to great distances (> 200 Re). The high latitude magnetosphere is filled with plasma as the solar wind passes the Earth. The flow of plasma into the magnetosphere increases with additional turbulence, density and speed in the solar wind. This flow is favoured by a southward component of the IMF, which can then directly connect to the high latitude geomagnetic field lines. The flow pattern of magnetospheric plasma is mainly from the magnetotail toward the Earth, around the Earth and back into the solar wind through the magnetopause on the day-side. In addition to moving perpendicular to the Earth's magnetic field, some magnetospheric plasma travels down along the Earth's magnetic field lines, gains additional energy and loses it to the atmosphere in the auroral zones. The cusps of the magnetosphere, separating geomagnetic field lines that close through the Earth from those that close remotely allow a small amount of solar wind to directly reach the top of the atmosphere, producing an auroral glow.
Learn More
Solar Winds Drive the Process
The solar wind is a stream of charged particles released from the upper atmosphere of the Sun, called the corona. This plasma consists of mostly electrons, protons and alpha particles with kinetic energy between 0.5 and 10 keV.
The dancing lights of the auroras provide spectacular views on the ground, but also capture the imagination of scientists who study incoming energy and particles from the sun. Auroras are one effect of such energetic particles, which can speed out from the sun both in a steady stream called the solar wind and due to giant eruptions known as coronal mass ejections or CMEs. After a trip toward Earth that can last two to three days, the solar particles and magnetic fields cause the release of particles already trapped near Earth, which in turn trigger reactions in the upper atmosphere in which oxygen and nitrogen molecules release photons of light. The result: the Northern and Southern lights.
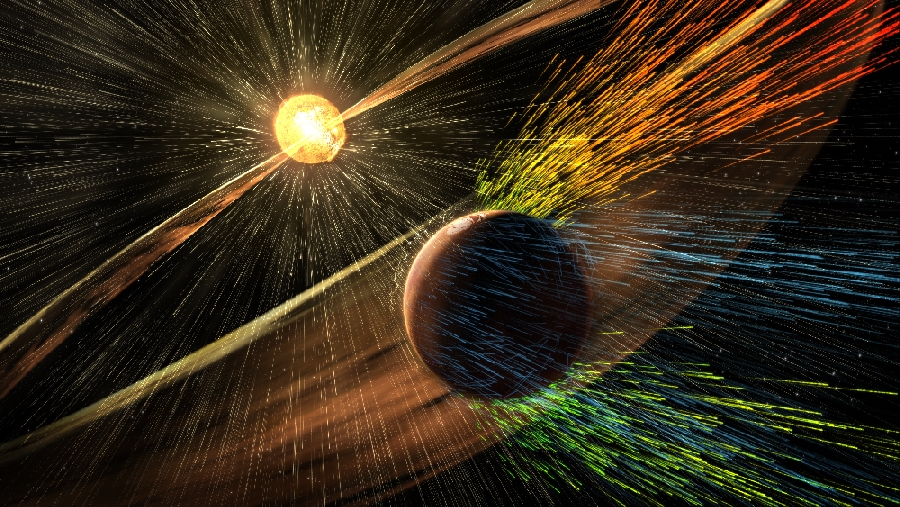
Above, we see the solar winds visualized, in this case stripping Mars of its atmosphere. Over time, solar wind has eroded Mars' atmosphere, transforming the planet into a wet and warm environment capable of supporting life into the cold and desolate planet that we see today. Since Earth has a magnetic field, unlike Mars, it has retained the atmosphere over time.
Atmospheric Interactions Create the Lights
Auroras result from emissions of photons in the Earth's upper atmosphere, above 80 km (50 mi), from ionized nitrogen atoms regaining an electron, and oxygen atoms and nitrogen based molecules returning from an excited state to ground state. They are ionized or excited by the collision of particles precipitated into the atmosphere. Both incoming electrons and protons may be involved. Excitation energy is lost within the atmosphere by the emission of a photon, or by collision with another atom or molecule:
- oxygen emissions - green or orange-red, depending on the amount of energy absorbed.
- nitrogen emissions - blue or red; blue if the atom regains an electron after it has been ionized, red if returning to ground state from an excited state.
Oxygen is unusual in terms of its return to ground state: it can take three quarters of a second to emit green light and up to two minutes to emit red. Collisions with other atoms or molecules absorb the excitation energy and prevent emission. Because the highest atmosphere has a higher percentage of oxygen and is sparsely distributed such collisions are rare enough to allow time for oxygen to emit red. Collisions become more frequent progressing down into the atmosphere, so that red emissions do not have time to happen, and eventually even green light emissions are prevented. This is why there is a color differential with altitude; at high altitudes oxygen red dominates, then oxygen green and nitrogen blue/red, then finally nitrogen blue/red when collisions prevent oxygen from emitting anything. Green is the most common color. Then comes pink, a mixture of light green and red, followed by pure red, then yellow (a mixture of red and green), and finally, pure blue.



